A rapid regulatory system for high-quality clinical trials
Queensland has the highest level of international standards for clinical research.
Queensland and Australia adopt a fast and pragmatic regulatory pathway for clinical trials with no Investigational New Drug (IND) requirement.
The regulatory framework is highly favourable, allowing rapid entry into clinical trials through streamlined processes under the Clinical Trials Notification scheme (CTN). The CTN is administered by the Therapeutic Goods Administration (TGA).
Queensland's single ethical review process saves you time while delivering good governance. You only need one ethical approval across Australia's public health system.
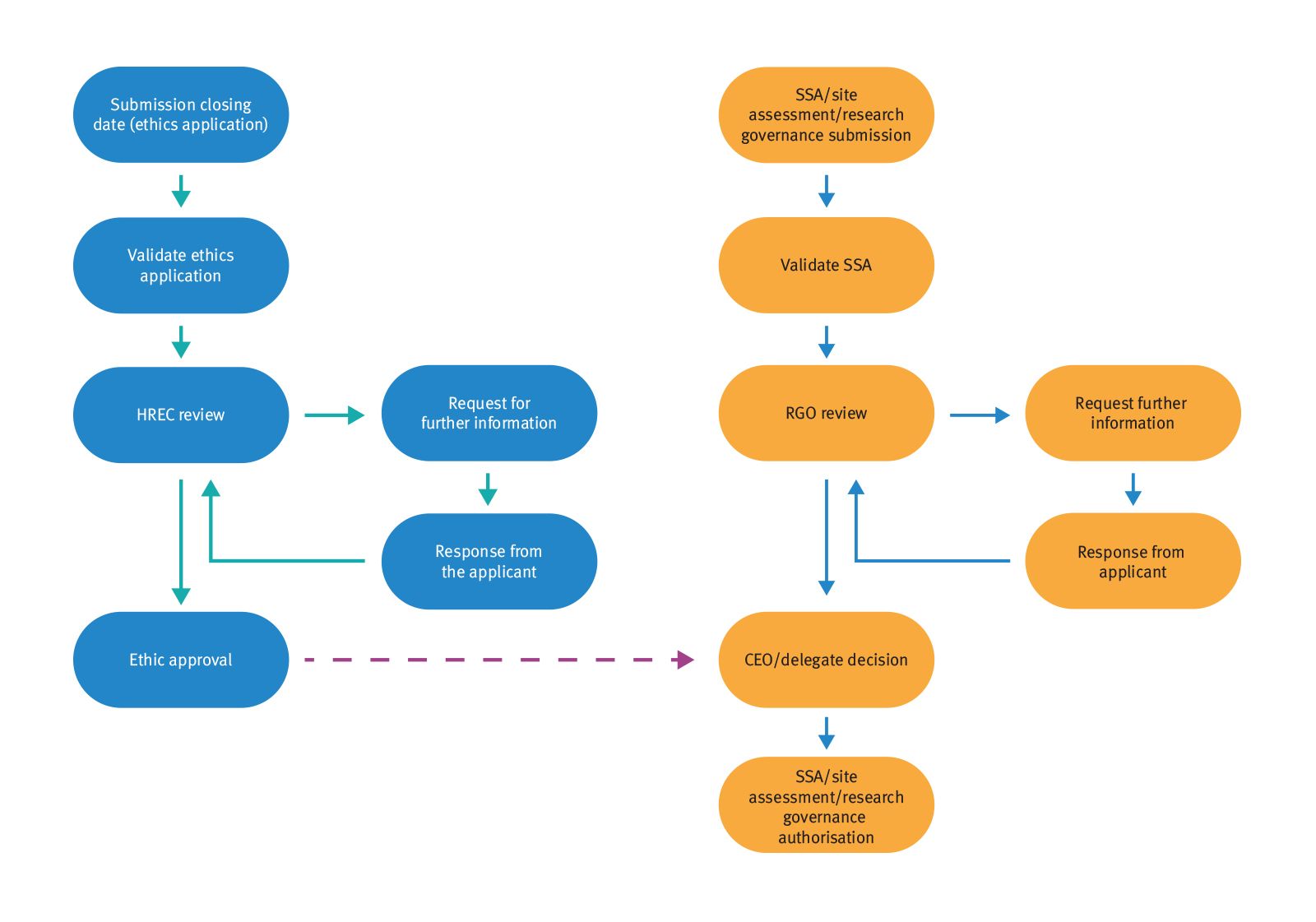
Queensland has access to 365 ethics committee meetings per year.
How are clinical trials regulated in Queensland?
Clinical trials in Australia are regulated on a number of levels under Commonwealth, state and territory legislation. The TGA is part of the Commonwealth Department of Health and is responsible for regulating medicines and medical devices—much like the Federal Drug Administration (FDA) in the United States.
The TGA oversees the inclusion of medicines and medical devices on the Australian Register of Therapeutic Goods (ARTG).
When medicines or medical devices for human use are imported, manufactured in Australia, supplied by a corporation, supplied interstate or to the Commonwealth or exported they must be included in the ARTG unless exempted.
Unapproved medicines and medical devices supplied in a clinical trial require an exemption under the Clinical Trial Notification Scheme (CTN) or through the Clinical Trial Application Scheme (CTA).
The TGA has strict compliance standards to ensure Australian products and services meet the highest global standards.
Australian research data is globally recognised, respected and supports international regulatory applications— including the FDA and the European Medicines Agency (EMA). This enables faster market entry and lowers costs.
How are regulatory burdens minimised?
Australia’s CTN scheme is a global benchmark for best practice in reducing the regulatory burden on clinical trial sponsors. This removes unnecessary duplication and saves sponsors a significant amount of time and money.
The majority of commercially sponsored clinical trials conducted in Australia are performed under the CTN scheme. All materials relating to a proposed clinical trial—including the trial protocol—are submitted directly to institutional ethics committees by researchers at the request of the relevant sponsor.
The ethics committee is solely responsible for assessing the:
- scientific validity of the trial design
- safety and efficacy of the medicine or device
- ethical acceptability of the trial process
- approval of the trial protocol
The institution where a clinical trial is held gives the final approval for the conduct of the trial at the site.
Under the CTN scheme, the TGA is simply notified of a clinical trial after it has received site approval and does not require further reviews of related data.
The TGA does have the authority to audit and enquire into the management of a clinical trial.
Get in touch today
Intellectual property protection
What are the major strengths of the Australian intellectual property system?
Australia has one of the strongest and most stable intellectual property systems in the world.
According to the International Property Rights Index, Australia’s intellectual property system ranks as the seventh most secure system in the world—out of 125 countries.1
Broadly deemed patentable subject matter
In Australia, patents are available for a wide variety of therapeutic inventions including:
- new formulations
- new active ingredients
- isolated forms of (therapeutically useful) natural products
- new methods of treatment
Inventions must meet the statutory requirements prescribed under the Patent Act 1990 (Cth) to be eligible for patent protection.
|
Patent term extensions In compliance with Article 33 of the World Trade Organisation’s Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS), Australia grants (standard) patent owners 20 years of protection. However, patent term extensions of up to five years may be available for patents relating to pharmaceutical substances if they satisfy statutory conditions.2 |
Data exclusivity Australia provides data exclusivity periods in connection with the registration of new therapeutic goods. Competitors are precluded from using proprietary information submitted to the TGA in support of their own application for an equivalent product. The product and information must meet the criteria for protection specified in the Therapeutic Goods Act 1989 (Cth). The protection applies for a five-year period commencing from the date the product is registered under the Act. |
Innovation patents
In Australia, an innovation patent lasts up to eight years, compared to 20 years for standard patents.
These patents are designed to protect inventions that do not meet the inventive threshold required for standard patents.
An innovation patent is a relatively fast and inexpensive way to protect intellectual property for a new medical device or pharmaceutical substance, method or process.3
1 2018 Intellectual Property Rights index: https://www.internationalpropertyrightsindex.org/country/australia
2 Section 70 of the Patents Act 1990 (Cth)
3 https://www.ipaustralia.gov.au/patents/understanding-patents/types-patents
